Have you ever wondered if there’s a liquid that can dissolve anything? It’s a question that has intrigued scientists and philosophers for centuries. In the realm of chemistry, this mystical liquid is often referred to as the “universal solvent.” While the concept of a substance that can dissolve everything seems magical, the reality is much more nuanced. In this exploration, we’ll delve into the fascinating world of solvents, unraveling the truth about the so-called “universal solvent,” and unraveling the mystery behind the phrase “circle the 4th letter of the first word.”
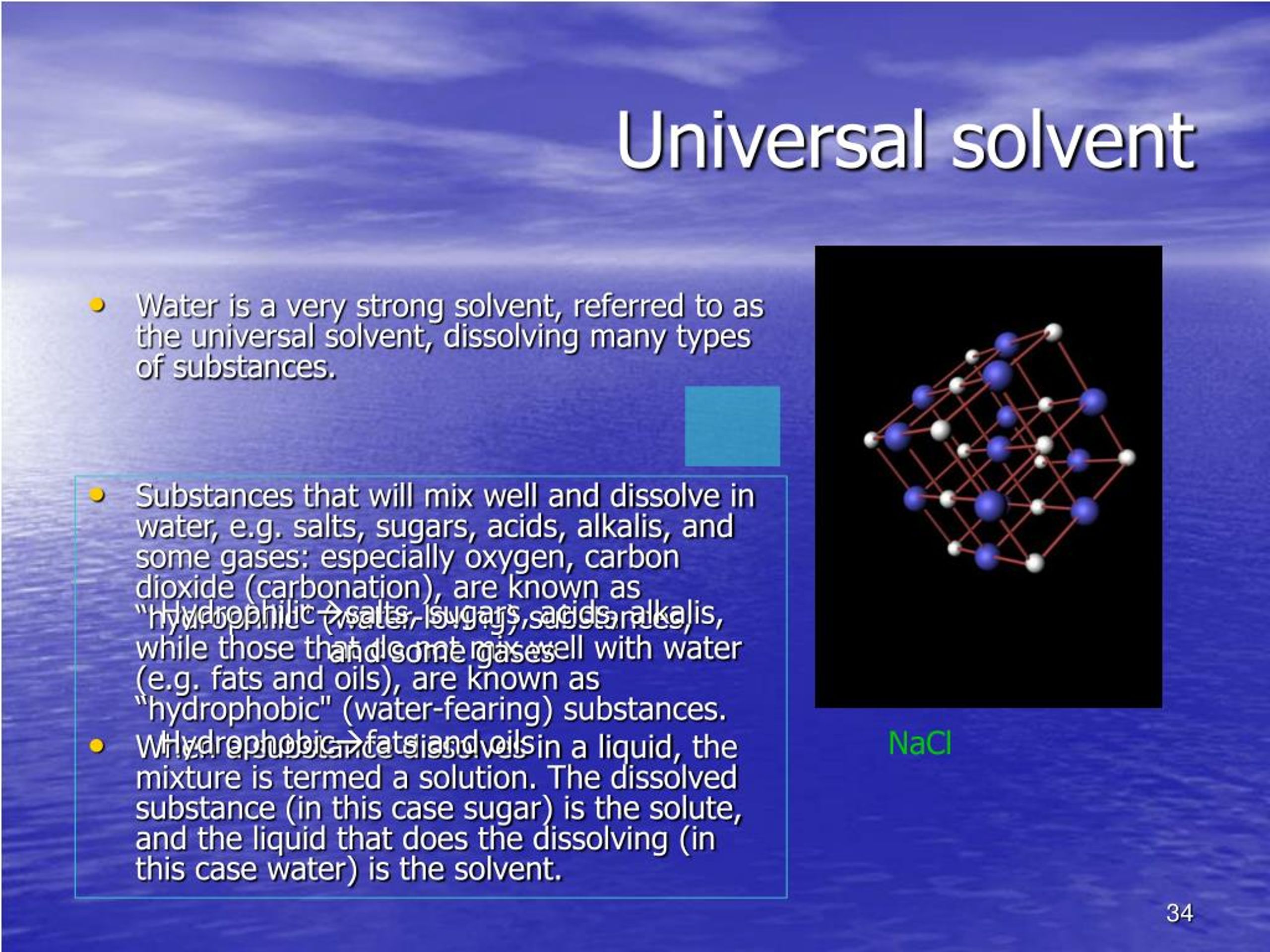
Image: www.slideserve.com
The quest for the perfect solvent has been a fascinating pursuit, driven by both practical and theoretical considerations. Imagine a liquid capable of dissolving all materials, rendering them into their constituent parts. Such a substance could revolutionize everything from waste management to materials science. But as we’ll discover, the pursuit of the universal solvent is fraught with challenges, ultimately revealing the complexity of chemical interactions and the boundaries of solubility.
Unveiling the Mystery of the “Universal Solvent”
The term “universal solvent” itself is a bit of a misnomer. No known substance can truly dissolve everything. The reason lies in the fundamental nature of chemical interactions. Solubility – the ability of a substance to dissolve in another – depends on the forces that hold molecules together. Like dissolves like: polar solvents, such as water, dissolve polar solutes (like salts), while nonpolar solvents, like oil, dissolve nonpolar solutes (like fats).
Water, often hailed as the “universal solvent,” is a potent example. Its high polarity allows it to dissolve a wide range of ionic compounds, such as salts, and polar organic molecules, such as sugars. However, it’s ineffective against nonpolar substances like fats and oils. In essence, no single solvent can conquer every material due to the diverse nature of chemical bonds.
The Mythological Origins: The Search for the Philosopher’s Stone
The idea of a universal solvent has deep roots in ancient alchemy. Alchemists sought the elusive “philosopher’s stone,” believed to possess the power to transmute base metals into gold, cure diseases, and dissolve any substance. This mythical stone was often associated with the universal solvent, underscoring the ancient fascination with the ultimate transformative power.
While alchemists’ experiments were based on mystical and spiritual beliefs, they paved the way for modern chemistry. The quest for the philosopher’s stone led to the development of numerous chemical processes and techniques, laying the groundwork for our understanding of elements, compounds, and reactions.
The Modern Perspective: The Importance of Solvents
Modern scientists and engineers recognize the importance of solvents not as a universal dissolving agent but as crucial tools for a wide range of applications. Solvents are used in everyday products like detergents, paints, and pharmaceuticals, and play critical roles in various industries, including manufacturing, agriculture, and energy production.
The choice of solvent depends on the specific application. For instance, water is used extensively as a solvent in cleaning products and industrial processes. Organic solvents, like acetone and ethanol, find applications in the production of paints, adhesives, and pharmaceuticals. The careful selection of solvents based on their properties and compatibility is crucial for achieving desired outcomes and minimizing environmental impact.

Image: www.geocities.ws
The Challenge: Understanding Solvents and Their Limitations
While solvents are essential tools, understanding their limitations is equally important. Some solvents can be harmful to human health and the environment. For example, volatile organic compounds (VOCs) released from certain solvents can contribute to air pollution and climate change.
Moreover, solvents can pose safety risks, especially when handled improperly. Flammable solvents can ignite easily, and some can be corrosive or toxic. It’s crucial to use solvents responsibly, following proper storage, handling, and disposal procedures. The pursuit of alternative solvents with improved safety and environmental profiles is an active area of research, driven by concerns for sustainability and human health.
Tips for Choosing and Using Solvents
Here are practical tips for selecting and using solvents safely and effectively:
- Choose the right solvent for the job: Consider the solubility of the substance you want to dissolve and the compatibility of the solvent with other materials involved.
- Use the minimum amount of solvent necessary: Reducing solvent consumption minimizes waste and reduces environmental impact.
- Handle solvents with care: Wear appropriate personal protective equipment (PPE), such as gloves, goggles, and respirators, to protect yourself from harmful exposure.
- Store solvents properly: Keep solvents in tightly sealed containers in a well-ventilated area away from heat and open flames.
- Dispose of solvents responsibly: Consult local regulations for proper disposal procedures.
Expert Advice: Navigating the World of Solvents
If you’re working with solvents, taking expert advice can make all the difference. Consider these recommendations:
- Consult safety data sheets (SDS): SDS provides detailed information about a solvent’s hazards, safe handling practices, and emergency protocols.
- Seek guidance from qualified professionals: Chemists, engineers, and safety experts can provide specific advice based on your specific needs and applications.
- Stay informed about industry standards and best practices: Keeping abreast of the latest regulations, advancements, and research can help you make informed decisions and minimize risks.
FAQ about Solvents
What is the difference between a solvent and a solute?
A solvent is a substance that dissolves another substance, called the solute. Think of water dissolving sugar; water is the solvent, and sugar is the solute.
What is the role of solvents in chemical reactions?
Solvents provide a medium for chemical reactions to occur. They can influence reaction rates and product formation by affecting the interactions between reactants.
What are some examples of common solvents?
Common solvents include water, acetone, ethanol, methanol, toluene, and hexane.
Are all solvents harmful to the environment?
No, not all solvents are harmful. Some solvents are biodegradable and have minimal environmental impact. However, many solvents can still pose risks if not handled or disposed of properly.
What are the future trends in solvent research?
Current trends in solvent research include developing greener and more sustainable solvents, such as ionic liquids and supercritical fluids, as well as exploring new solvent technologies for specific applications like carbon capture and sequestration.
Universal Solvent Circle The 4th Letter Of The 1st Word
Conclusion: The Enduring Quest for Understanding
The search for the universal solvent, though ultimately unattainable, has been a driving force in scientific exploration. It underscores the human desire to unravel the mysteries of the natural world and harness its power for practical applications. While the perfect dissolving agent may remain elusive, our understanding of solvents continues to evolve, leading to innovations in various fields and a greater appreciation for the intricate interplay of molecules and their interactions.
Are you interested in learning more about specific solvents, their properties, applications, and safety considerations? We encourage you to delve deeper into the fascinating world of solvents and explore the ever-expanding frontiers of chemical science. Share your thoughts and questions in the comments below!





