Have you ever wondered why some reactions happen in a flash while others seem to take forever? Or perhaps you’ve witnessed how adding a pinch of something can drastically change how quickly a reaction proceeds? These intriguing questions lead us to the fascinating world of chemical reaction rates and the myriad factors that govern them. Understanding these factors is vital, not only for scientists and chemists, but for anyone who interacts with the world around them, from aspiring bakers to curious gardeners.
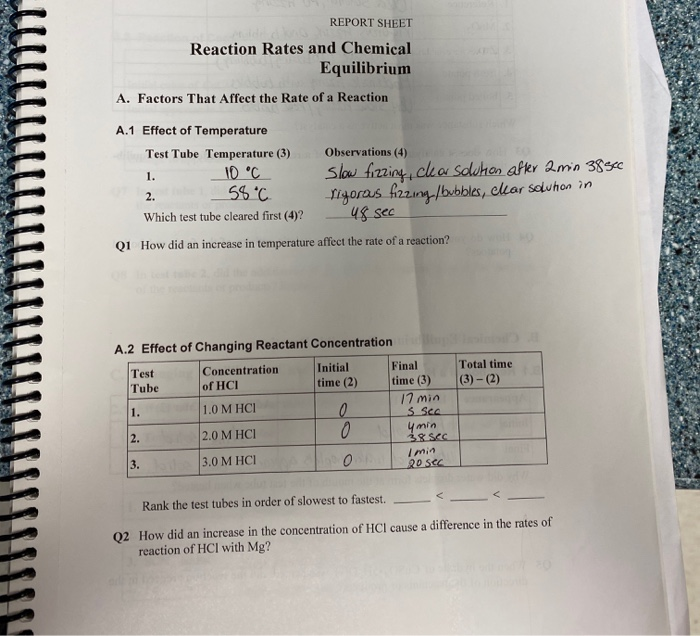
Image: www.chegg.com
Chemical reactions are the fundamental building blocks of our universe. They drive everything from the rusting of a metal object to the intricate processes within our bodies. The rate at which these reactions occur determines how fast or slow a change takes place. In this exploration, we will delve into the key factors that influence the speed of chemical reactions, unveiling the mechanisms behind their dynamic behavior.
The Fundamental Players: Concentration, Temperature, and Surface Area
Imagine a bustling marketplace overflowing with people. The more people there are, the more likely they are to bump into each other, right? Similarly, the concentration of reactants in a chemical reaction directly impacts the rate. Higher concentrations mean more frequent collisions between reactant molecules, leading to more frequent successful reactions. Just like a crowded street, where chance encounters are more frequent, a higher concentration of reactants creates a greater probability for collisions and subsequent reactions.
Temperature, the measure of heat, plays a pivotal role in the speed of chemical reactions. Think of a fire. The hotter the fire, the faster the wood burns. This is because higher temperatures infuse molecules with more kinetic energy, causing them to move faster and collide more often. These energetic collisions are more likely to overcome the activation energy barrier, the minimum energy required for a reaction to occur. In essence, higher temperatures provide the reactants with more opportunities to react.
Surface area is another crucial factor that can dramatically affect reaction rates. Imagine trying to dissolve a sugar cube versus a spoonful of granulated sugar. The granulated sugar dissolves much faster because it has a larger surface area exposed to the solvent. Similarly, in a chemical reaction, reactants with a greater surface area offer more points of contact for collisions, leading to a faster rate of reaction.
Catalysts: The Unsung Heroes of Chemical Reactions
Life without catalysts would be a much slower, less efficient affair. Catalysts are like masterminds, orchestrating chemical reactions without being consumed in the process. They provide an alternate pathway for the reaction, one with a lower activation energy. Think of a mountain pass. It’s much easier to traverse the mountain using a pass than climbing directly over the peak. Catalysts act like this pass, allowing the reactants to react at a much faster rate.
Enzymes, nature’s catalysts, are a testament to the power of catalysis. They orchestrate the complex biochemical reactions within our bodies, enabling us to digest food, breathe, and think. Catalysts also play a crucial role in industrial processes, accelerating reactions and minimizing energy consumption. The Haber-Bosch process, which synthesizes ammonia, a key ingredient in fertilizer, relies on catalysts to enable this vital reaction at a practical rate.
The Importance of Understanding Chemical Reaction Rates
Understanding the factors that influence chemical reaction rates is not just a scientific pursuit; it is a gateway to unlocking a world of possibilities in various fields. In medicine, it helps us develop drugs and therapies by tailoring the rates of biochemical reactions. In environmental science, it allows us to understand and mitigate pollution by studying the rates of reactions involved in environmental processes. In industries, it drives innovation by optimizing manufacturing processes and developing new materials.
Consider the humble refrigerator. It keeps our food fresh by slowing down the rate of spoilage, a chemical reaction that involves the breakdown of food molecules. Farmers, on the other hand, harness the power of chemical reactions by using fertilizers that accelerate the growth of plants. Indeed, the world around us is a testament to the dynamic nature of chemical reactions and the vital role of their rates in shaping our world.
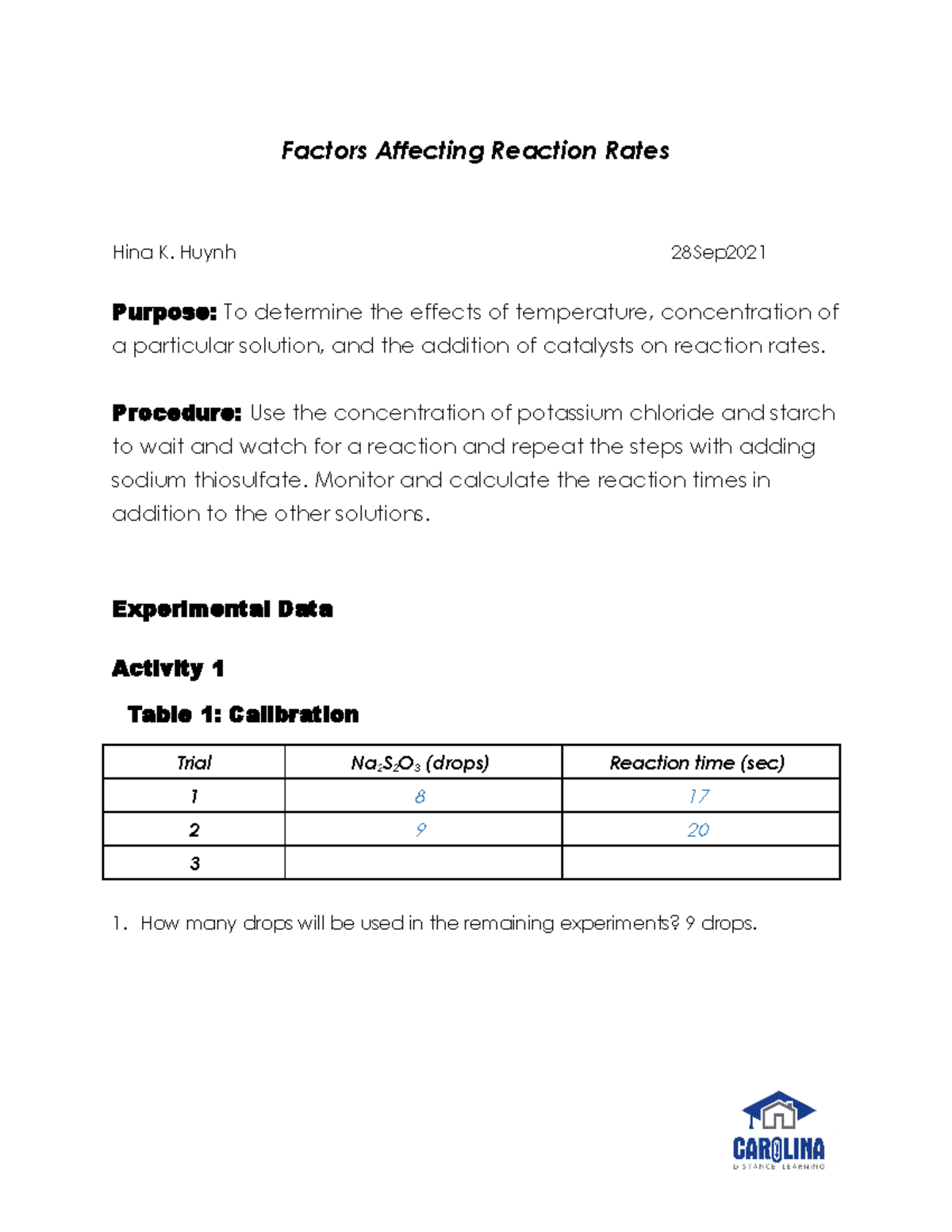
Image: www.studocu.com
Tips for Conducting a Successful Reaction Rate Lab Report
- Plan your experiments carefully: Start with a clear objective and a well-designed experiment to isolate and measure the effect of each factor on the reaction rate.
- Control the variables: Keep all other factors constant except the one you are investigating. This ensures that any observed change in the rate is due solely to the factor you are manipulating.
- Measure the rate accurately: Use reliable methods to measure the rate of the reaction, such as monitoring the change in concentration over time or the rate of gas evolution.
- Analyze your data thoughtfully: Plot your data and draw meaningful conclusions. Use statistical tools to ensure the significance of your observations.
Factors Affecting The Rate Of A Chemical Reaction Lab Report
Conclusion: A World of Reactions
The world of chemistry is a vibrant tapestry of intricate reactions, each governed by the interplay of various factors. By understanding the key factors that affect the rates of these reactions, we gain invaluable insight into the mechanisms shaping our universe, from the tiniest molecules to the vastest of stars. So next time you witness a chemical transformation, pause for a moment to appreciate the intricate dance of molecules, driven by the very principles we have explored. And remember, even in the seemingly mundane, lies a universe of fascinating chemical reactions, forever unfolding before our very eyes.





