Imagine walking barefoot on the hot sand of a beach. The scorching heat sears your skin, a stark contrast to the coolness of the ocean water just a few steps away. Why is the sand so much hotter than the water, even though they have been basking in the same sun? The answer lies in a fascinating property called specific heat. It’s the key to understanding why some materials heat up or cool down faster than others, and it’s a fundamental concept in physics and chemistry. This guide will explore the captivating world of specific heat, providing you with a comprehensive understanding through engaging explanations, insightful examples, and practical worksheets with answers to help you master this essential concept.

Image: studylib.net
Specific heat is a measure of how much energy is needed to raise the temperature of a substance by a certain amount. It plays a crucial role in countless aspects of our lives, from cooking to weather patterns. Whether you’re a student delving into the world of science, a curious individual seeking a deeper understanding of the world around you, or a professional seeking to apply this knowledge in your field, this guide will serve as your comprehensive companion to unlock the fascinating secrets of specific heat.
Delving into the Depths of Specific Heat
To understand specific heat, we must first understand the basic concept of heat. Heat is a form of energy that flows from a hotter object to a cooler object when they are in contact. This energy transfer, measured in joules, causes the temperature of the cooler object to rise and the temperature of the hotter object to decrease until they reach thermal equilibrium. It’s a fundamental principle that governs the exchange of heat energy, driving processes ranging from cooking a meal to warming our homes.
Specific heat, represented by the symbol ‘c’, is a material property that quantifies its resistance to temperature change. It’s defined as the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius. Each substance has a unique specific heat value, reflecting its inherent capacity to absorb and store heat. For example, water has a high specific heat capacity, meaning it takes a lot of energy to raise its temperature. This is why oceans act as massive heat reservoirs, moderating coastal temperatures. Conversely, metals have low specific heat capacities, meaning they heat up quickly and cool down quickly. This is why a metal spoon in hot soup will become hot to the touch much faster than a wooden spoon of the same size.
The Importance of Specific Heat in Our World
Specific heat impacts various aspects of our lives, playing a vital role in diverse fields. Here are a few examples:
1. Cooking: Understanding specific heat is essential in the culinary world. Different ingredients have different specific heat values. Water, for instance, has a relatively high specific heat value, meaning it takes more energy to raise its temperature than other ingredients like oil or fat. This is why it takes longer to boil water than to heat oil.
2. Meteorology: Specific heat is crucial in understanding weather patterns. The oceans, covering about 71% of the Earth’s surface, have a high specific heat capacity, acting as enormous heat sinks. This regulates our planet’s climate, moderating temperature changes and creating stable weather patterns.
3. Engineering: Engineers utilize specific heat knowledge in designing various systems. For example, high-heat-resistance materials like ceramics are often used in engines and turbines due to their high specific heat capacities. This allows them to withstand extreme temperatures without compromising performance.
Specific Heat: A Hands-On Exploration
To make understanding specific heat more tangible, let’s delve into a practical example. Imagine a glass of water and a metal pan, both placed in a hot oven. The metal pan will become hot to the touch much faster than the glass of water. This is because the metal pan has a lower specific heat capacity than water. It takes less energy to raise the temperature of the metal pan, making it heat up quicker.
Now, let’s consider a scenario involving a hot cup of coffee. If you want to cool it down quickly, adding ice is a common practice. This works because ice has a relatively low specific heat capacity, meaning it absorbs heat energy quickly and readily melts. During this process, ice absorbs heat from the hot coffee, reducing its temperature significantly. On the other hand, if you add cold water to the coffee, it takes longer to cool down. This is because cold water has a higher specific heat value and absorbs less heat from the coffee, resulting in a slower cooling process.
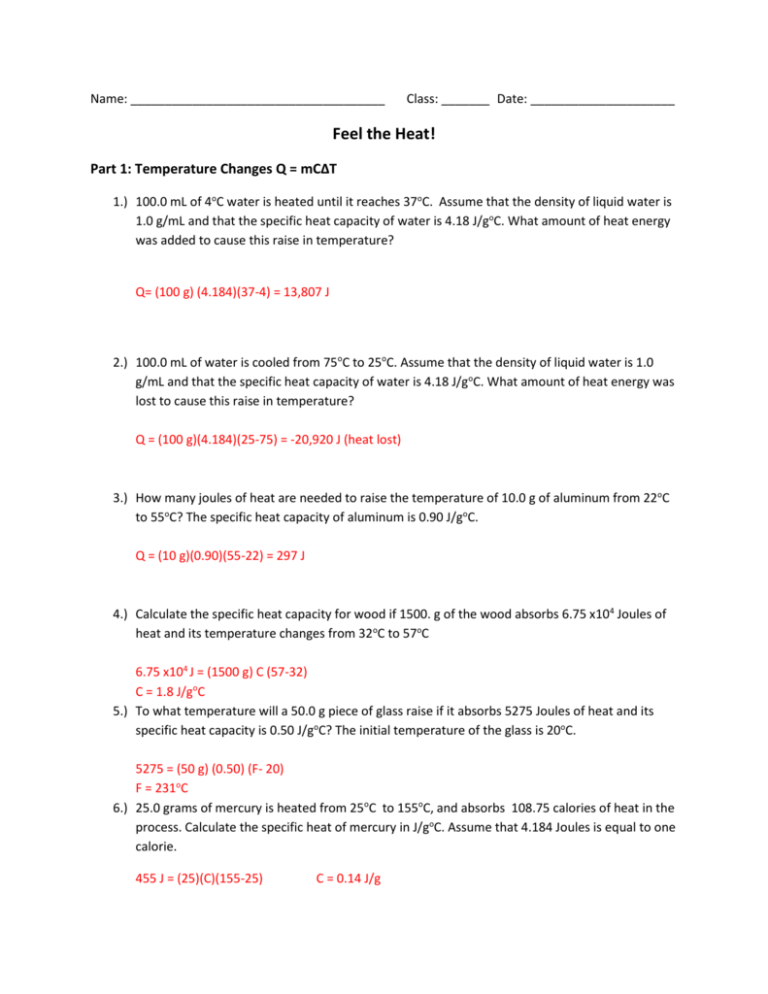
Image: answermediabrandt.z19.web.core.windows.net
Master the Math: Specific Heat Calculations
Specific heat can be calculated using the following formula:
Q = mcΔT
Where:
- Q represents the amount of heat energy transferred (measured in joules)
- m represents the mass of the substance (measured in grams)
- c represents the specific heat capacity of the substance (measured in joules per gram per degree Celsius)
- ΔT represents the change in temperature (measured in degrees Celsius)
Specific Heat Worksheet Answers: Your Learning Companion
To further solidify your grasp of specific heat, we’ve incorporated worksheets with answers, offering valuable practice and reinforcement. These worksheets are designed to help you understand the relationship between heat energy, specific heat, mass, and temperature changes. They cover various applications and scenarios, ranging from calculating the heat energy needed to raise the temperature of a specific substance to determining specific heat values based on experimental data.
For example, one worksheet might ask you to calculate the amount of heat energy required to raise the temperature of 100 grams of water from 20°C to 80°C, given that the specific heat capacity of water is 4.18 J/g°C. The answer, which you can find in the answer key, involves applying the formula and plugging in the appropriate values. The worksheets provide a step-by-step approach to problem-solving, guiding you through calculations and enhancing your conceptual understanding.
Expert Insights: Unveiling the Secrets of Specific Heat
Experts in physics and chemistry often emphasize the importance of understanding specific heat in various applications. For instance, in the field of materials science, specific heat considerations play a vital role in developing materials with specific properties suited for particular applications. For example, materials with high specific heat capacities are often used in heat sinks, devices that dissipate excess heat from electronic components, ensuring optimal performance.
In the realm of climate science, experts highlight the importance of specific heat in understanding climate change and its effects. The oceans’ high specific heat capacity significantly impacts global climate patterns. As temperatures rise due to climate change, the oceans absorb vast amounts of heat, moderating the rate of warming on land. However, the increasing heat absorption by the oceans is causing changes in oceanic currents and contributing to sea level rise, representing a complex challenge for our planet.
Specific Heat Worksheet With Answers Pdf
Specific Heat: A Journey of Discovery
This comprehensive journey into the world of specific heat has unveiled its foundational concepts, practical applications, and the fascinating ways it shapes our world. We’ve explored how specific heat governs the exchange of heat energy, influencing everything from cooking methods to global climate patterns. Through engaging explanations, illustrative examples, and practical worksheets, we’ve empowered you with the tools and knowledge to confidently understand and apply this essential concept.
Whether you’re pursuing scientific endeavors, seeking a deeper understanding of the world around you, or simply curious about the intricate workings of our universe, specific heat represents a fascinating window into the fundamental principles that govern our existence. Let us know your insights and experiences with this fascinating concept in the comments below.





