Remember that time you were in science class and your teacher started talking about atoms and ions? Maybe you sat there, completely bewildered, wondering why you needed to know about these tiny particles. But what if I told you that understanding atoms and ions is the key to understanding just about everything around you? From the water you drink to the air you breathe, even the very ground you walk on, atoms and ions are the building blocks of our world. They are the microscopic players in a captivating cosmic drama, and once you truly understand their roles, you’ll see the world in a whole new light.

Image: classdbdaecher.z13.web.core.windows.net
This article is your guide to mastering the intricacies of atoms and ions. We’ll explore the answers to that atoms vs. ions worksheet that’s been puzzling you, and shed light on these foundational concepts. You’ll discover how these particles, so incredibly small, influence the world we experience every single day. Hold on tight, because we’re about to embark on an exciting microscopic journey!
Delving into the Atom: Tiny Building Blocks of Everything
Let’s start our journey by getting to know the very foundation of matter, the atom. Picture a miniature solar system, with a dense, positively charged nucleus at its center. This nucleus is made up of even smaller particles called protons and neutrons. The protons, carrying a positive charge, are like the sun, giving the nucleus its identity. The neutrons, neutral and stable, complement the protons, adding to the nucleus’s mass. Like tiny planets orbiting the sun, negatively charged electrons whiz around the nucleus in specific energy levels. These electrons determine how the atom interacts with the outside world and how it bonds with other atoms.
Think of it this way: Each atom has a unique “fingerprint” based on the number of protons in its nucleus. This number, called the atomic number, is like a secret code that defines the element the atom belongs to. For instance, an atom with one proton is hydrogen, six protons form carbon, and eight protons create oxygen. So, all atoms of a particular element have the same number of protons!
Atoms in Action: Where the Fun Really Begins
Now that we know about atoms, let’s step up the game and learn about ions. The word “ion” comes from the Greek word “ion,” meaning “going.” These charged particles are formed when atoms gain or lose electrons. Imagine an atom with all its electrons neatly orbiting its nucleus. If this atom loses an electron, it becomes positively charged and is transformed into a positively charged ion, often called a cation. Similarly, if an atom gains an electron, it becomes negatively charged, forming a negatively charged ion, known as an anion.
Think of it like this: If a neutral atom loses a negatively charged electron, it becomes positively charged, like taking away a minus sign from a mathematical equation. On the other hand, if a neutral atom gains a negatively charged electron, it becomes negatively charged, like adding a minus sign to the equation. This simple addition or subtraction of electrons changes the atom’s whole personality!
The Big Picture: Understanding How Atoms and Ions Shape Our World
You might think this is just a bunch of abstract theories, but the reality is, atoms and ions are constantly at work, shaping our world in fascinating ways. Let’s look at some examples:
-
Water: You’re probably familiar with the chemical formula for water: H2O. This formula tells us that each water molecule is made up of two hydrogen atoms and one oxygen atom. But here’s the catch, these atoms are not neutral! The hydrogen atoms each lose an electron, becoming positively charged ions, while the oxygen atom gains two electrons, becoming negatively charged. This combination of opposite charges creates a strong bond, holding the water molecule together.
-
Salts: Table salt, a common ingredient in our kitchens, is another fantastic example. It is formed from the combination of sodium (Na), a positively charged ion, and chlorine (Cl), a negatively charged ion. The opposite charges attract each other, forming the iconic salt crystal.
-
Electricity: The flow of electricity depends on the movement of electrons. These electrons can move freely through conductors, like metals, because their outer electrons are loosely bound and can easily jump from one atom to another. This free flow of electrons enables electricity to travel through wires, power our devices, and light up our homes.
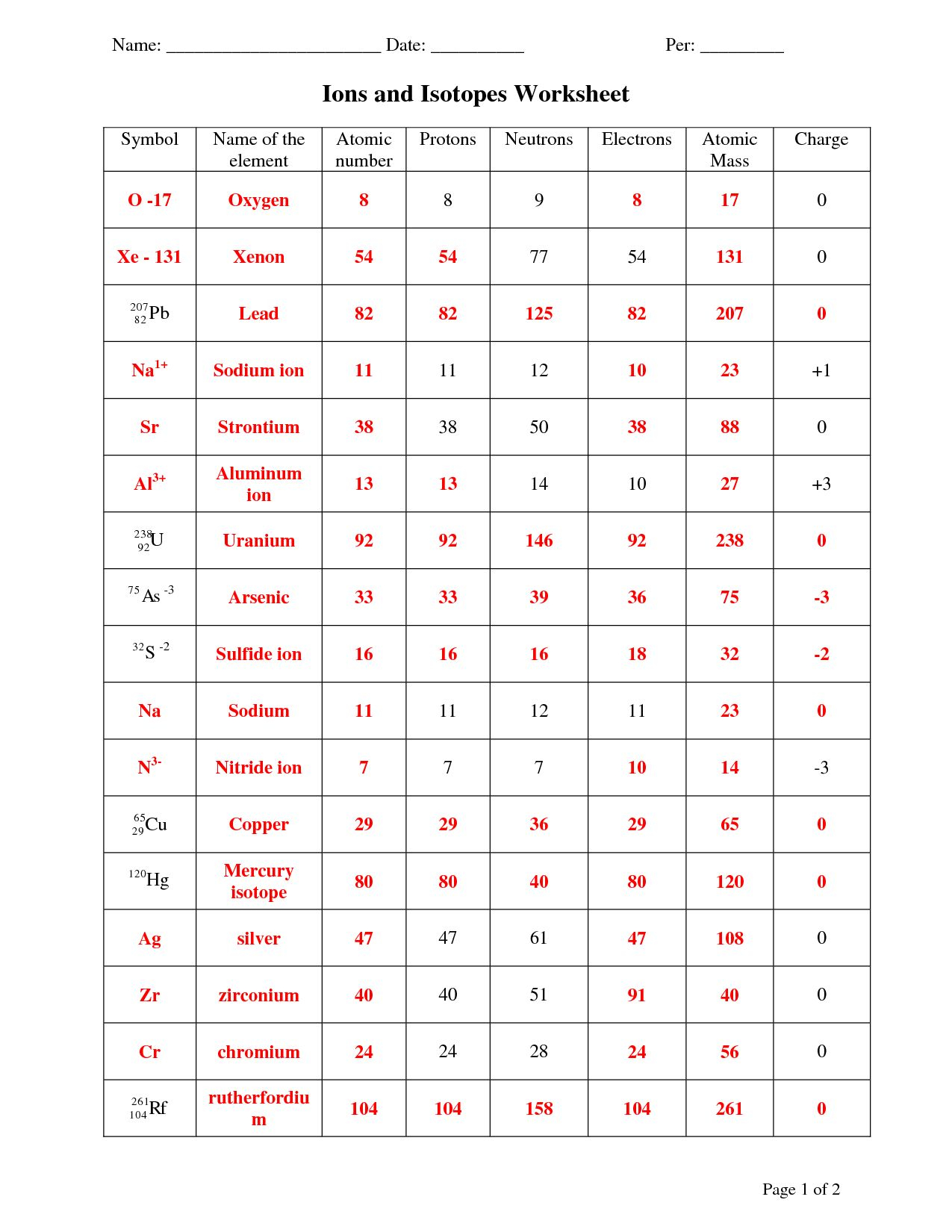
Image: learninglistlang.z19.web.core.windows.net
The Magic of Ions: Unveiling the Powers of Tiny Particles
From the water we drink to the air we breathe, ions play a vital role in processes fundamental to life.
-
Biological processes: Ions are crucial for a wide range of biological processes, ranging from nerve impulses to muscle contraction. For example, the sodium-potassium pump, a vital mechanism in our cells, works by moving sodium and potassium ions across cell membranes. This movement generates electrical signals that facilitate nerve impulses and muscle contractions.
-
Environmental processes: Ions are involved in many environmental processes. For instance, they control water quality and influence climate. The dissolution of minerals in rainwater forms ions that contribute to soil fertility.
-
Chemistry: Ions are central to the field of chemistry, enabling chemical reactions and the formation of compounds. Understanding how atoms form ions is crucial for comprehending the world around us.
Atoms vs. Ions Worksheet Answer Key: Unlocking the Solutions
Now that we understand the building blocks of atoms and ions, let’s tackle the atoms vs. ions worksheet. Here’s a breakdown of the key concepts to help you navigate through those tricky questions:
1. Defining the Terms:
- Atom: The basic unit of an element, composed of a nucleus with protons and neutrons, and electrons orbiting around it.
- Ion: An atom that has gained or lost electrons, making it electrically charged.
2. Identifying Ions:
- Look for the presence of a charge, indicated by a superscript plus (+) sign (for cations) or a superscript minus (-) sign (for anions).
- Remember: A positively charged ion (cation) has lost electrons, while a negatively charged ion (anion) has gained electrons.
- Focus on the number of protons and electrons. For example, a neutral sodium atom has 11 protons and 11 electrons. If it loses one electron, it becomes a sodium ion (Na+) with 11 protons and 10 electrons.
3. Ions in Chemical Formulas:
- Pay attention to the charges of ions and how they combine to form electrically neutral compounds.
- For example, in sodium chloride (NaCl), the sodium ion (Na+) has a +1 charge, while the chloride ion (Cl-) has a -1 charge. They combine to form a neutral compound.
- Remember that the total positive charge must balance the total negative charge in a compound.
4. Exploring Ion Properties:
- Ions are generally smaller than atoms because they have lost or gained electrons, changing the attractive forces within the atom.
- Ions exhibit different properties from their neutral atom counterparts due to their electrical charges.
Atoms Vs Ions Worksheet Answer Key
Mastering the Microscopic World: Your Key to Understanding the Universe
You’ve successfully embarked on a journey through the fascinating world of atoms and ions. Armed with this knowledge, you’ll find yourself understanding the world around you in a whole new light. You’ll be able to decipher the language of chemistry, comprehend the mechanisms underpinning life, and appreciate the power of these tiny particles that shape our universe. The next time you encounter an atoms vs. ions worksheet, it won’t seem daunting. Instead, you’ll approach it with confidence and a newfound appreciation for the microscopic forces that govern our world. So go forth, explore, and let the wonders of atoms and ions inspire you!





