Have you ever wondered how scientists can predict the amount of heat released or absorbed during a chemical reaction without even performing the reaction in a lab? The answer lies in a powerful tool called Hess’s Law, a fundamental principle in chemistry that simplifies the calculation of enthalpy changes by breaking down complex reactions into simpler steps. Today, we’ll delve into Hess’s Law and tackle the challenges posed by Chem Worksheet 16-5.

Image: www.coursehero.com
Whether you’re struggling with the concept of enthalpy change or seeking to master Hess’s Law for your chemistry exams, this article will be your guide to unlocking the secrets of this invaluable tool. We’ll walk through step-by-step solutions to the problems in Worksheet 16-5, providing a deeper understanding of the principles behind each calculation.
Introducing Hess’s Law: A Powerful Tool for Enthalpy Change
Hess’s Law, named after the Swiss chemist Germain Henri Hess, states that the enthalpy change for a reaction is independent of the pathway taken. Essentially, it means that no matter how many steps a reaction is broken into, the total enthalpy change remains the same. This law simplifies the calculation of enthalpy changes for complex reactions, allowing us to determine the enthalpy change of a reaction by examining simpler, related reactions.
Imagine you’re climbing a mountain. You can choose to take a direct, steep route or a longer, winding path with multiple switchbacks. Regardless of the path you choose, the overall elevation gain remains the same – the difference in altitude between the starting point and the summit. Similarly, the enthalpy change for a chemical reaction remains constant, whether it occurs in one step or multiple steps.
Hess’s Law in Action: Solving Chem Worksheet 16-5
Let’s dive into Chem Worksheet 16-5 and see Hess’s Law in action. Each problem is essentially a chemical puzzle – you’re given a target reaction and a set of equations representing simpler reactions. The challenge lies in manipulating these simpler reactions to arrive at the target reaction and then calculating the enthalpy change.
Here’s a breakdown of the steps involved in solving Hess’s Law problems:
1. Identify the Target Reaction: Clearly define the reaction for which you want to determine the enthalpy change. This is often expressed in the problem statement.
2. Analyze the Given Equations: Examine the equations provided. Look for the reactants and products of the target reaction within these equations.
3. Manipulate the Equations: You can manipulate the given equations by:
- Reversing the equation: If a reaction is reversed, the sign of the enthalpy change is also reversed.
- Multiplying the equation by a factor: Multiplying the equation by a factor also multiplies the enthalpy change by the same factor.
- Adding or Subtracting Equations: The enthalpy change for the resulting equation is the sum or difference of the enthalpy changes of the individual equations.
4. Combine Equations to Achieve the Target Reaction: By carefully manipulating and combining the given equations, strategically add, subtract, or reverse them to obtain the target reaction.
5. Calculate the Enthalpy Change: Once you’ve achieved the target reaction, sum the enthalpy changes of the manipulated equations to determine the enthalpy change for the target reaction.
The Essence of Hess’s Law: Unveiling Enthalpy Change
Hess’s Law empowers us to predict enthalpy changes without actually conducting experiments, saving time and resources. Its application extends beyond academic exercises, playing a crucial role in various chemical and engineering fields, from designing new chemical processes to understanding combustion reactions.
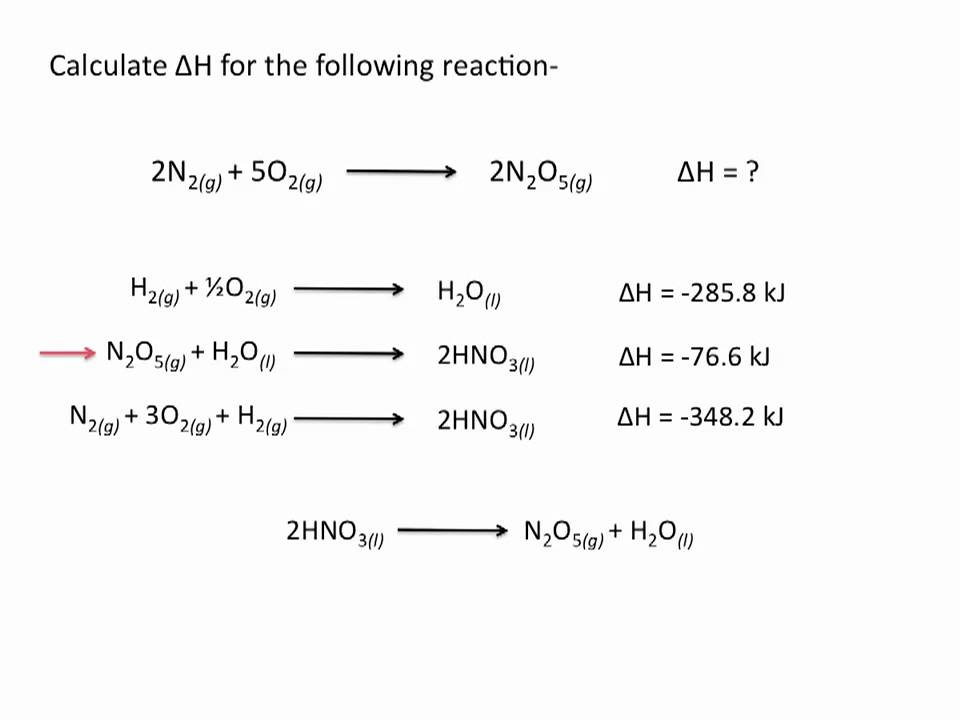
Image: printablelibfleischer.z19.web.core.windows.net
Hess’s Law in Real-World Applications: Beyond the Classroom
Beyond the pages of your chemistry textbook, Hess’s Law plays a vital role in numerous fields. Here are a few examples:
- Industrial Chemistry: In industries that rely on chemical processes, Hess’s Law helps engineers optimize reaction conditions, minimizing energy consumption and maximizing production efficiency.
- Environmental Science: Hess’s Law assists in understanding the energetics of complex chemical reactions occurring in the environment, such as in atmospheric processes and combustion reactions.
- Pharmaceutical Industry: In drug development, Hess’s Law helps predict the enthalpy changes during drug synthesis and formulation, ensuring efficient and safe production processes.
Hess’S Law Chem Worksheet 16-5 Answers
Key Takeaways: Mastering the Art of Enthalpy Change
Hess’s Law is a powerful tool for calculating enthalpy changes, enabling us to predict the energy released or absorbed in chemical reactions. By mastering this law, you gain a deeper understanding of chemical reactions and their energy profiles.
Remember, practice is key to mastering these concepts. As you work through Chem Worksheet 16-5, take your time to understand each step, and don’t hesitate to seek help from your teacher, tutor, or online resources if needed. Keep in mind, the journey to understanding Hess’s Law is an exciting exploration of the intricate world of chemical reactions and energy transformations. And remember, with every problem solved, your understanding of this powerful law will grow stronger.





