Imagine yourself, weightless and serene, gliding through a kaleidoscope of coral and marine life. The silence is broken only by the rhythmic hiss of your breathing regulator, a constant reminder of the unseen world that sustains you – the world of gas laws. For scuba divers, understanding the principles of gas laws is not just a scientific curiosity, it’s a matter of life and death. This article will dive deep into the fascinating and essential relationship between these natural laws and the sport of scuba diving.
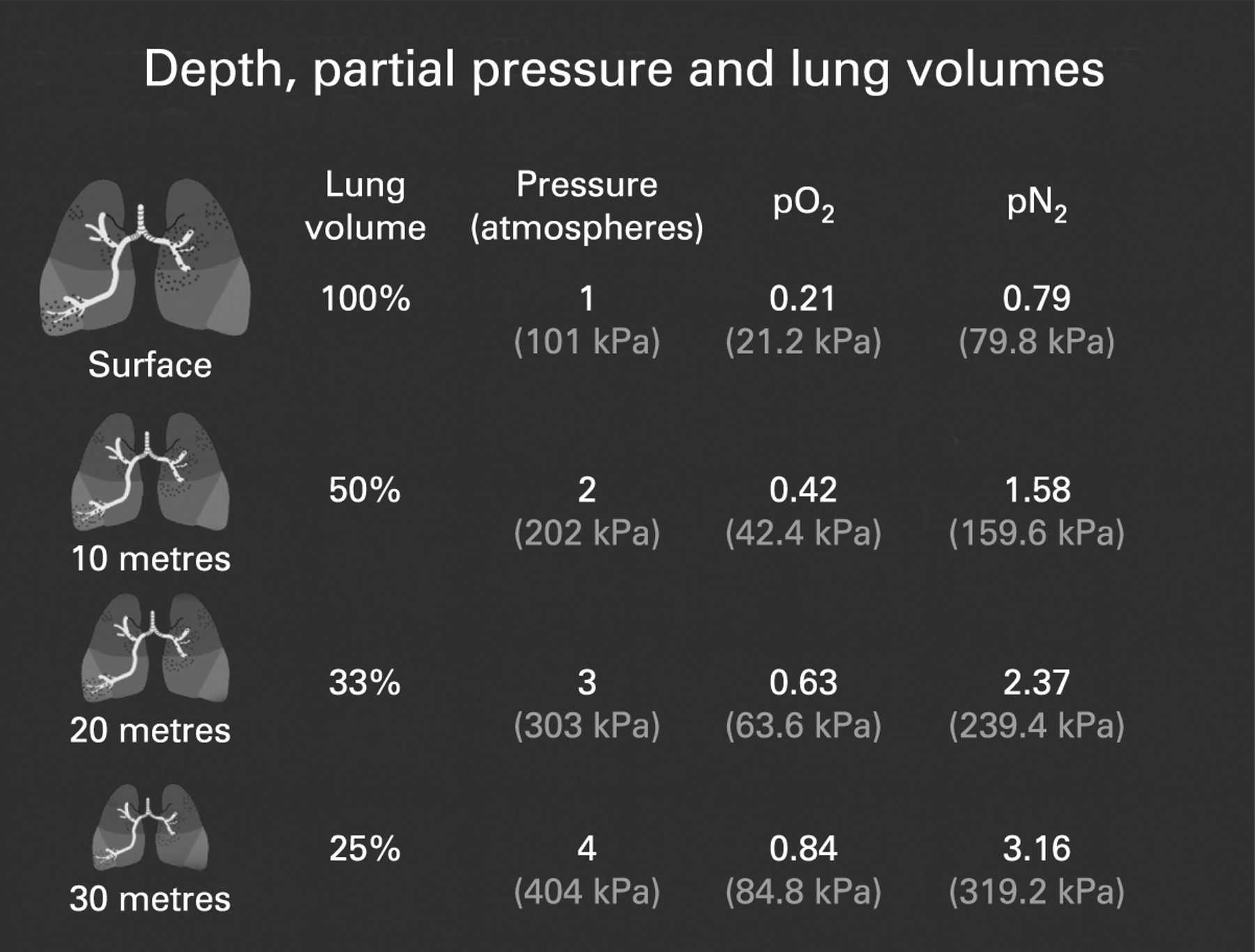
Image: www.semesprit.com
Knowing how gas behaves under pressure is a fundamental skill for anyone venturing beneath the waves. From calculating the amount of air you need for a dive to understanding the risks of decompression sickness, the gas laws dictate the very rules of engagement in the underwater world. We’ll explore the core principles that govern gas behavior, break down their implications for divers, and ultimately empower you with the knowledge to enjoy safe and exhilarating scuba adventures.
Diving into the Depths of Gas Laws
The gas laws aren’t just abstract equations in a textbook. They are the invisible forces that govern the very air we breathe, both on land and underwater. So, let’s start with the foundational concepts:
Boyle’s Law: The Inverse Proportionality
Boyle’s Law states that the volume of a gas is inversely proportional to its pressure. Think of it like squeezing a balloon. As you squeeze, the pressure inside increases, and the volume of the balloon decreases. In the underwater world, this means as a diver descends, the pressure surrounding them increases, and the volume of the air in their lungs shrinks.
Dalton’s Law: Partial Pressures
Dalton’s Law explains that the total pressure of a gas mixture is equal to the sum of the partial pressures of each gas in the mixture. This means that each gas in a mixture, like the air we breathe, exerts its own individual pressure. In scuba diving, this is particularly relevant to the pressure of oxygen in the air mixture, which can become dangerously high at depth.

Image: answerlistwade123.z13.web.core.windows.net
Henry’s Law: Gases in Liquids
Henry’s Law describes the solubility of gases in liquids, specifically how gases dissolve into liquids in proportion to their partial pressure. For divers, this means that as they descend deeper, the partial pressure of nitrogen in the air they breathe increases, and more nitrogen dissolves into their blood and tissues. This can lead to nitrogen narcosis, also known as “the rapture of the deep,” which affects judgment and coordination.
Charles’s Law: Volume and Temperature
Charles’s Law dictates that the volume of a gas is proportional to its absolute temperature, meaning that a gas expands when heated and contracts when cooled. While this law is less directly relevant to the diving experience itself, it plays a role in understanding how gas behaves in equipment like scuba tanks.
Deeper Insights for Deeper Dives
Now that we have a grasp of the fundamental gas laws, let’s dive into their practical applications for scuba divers:
Air Consumption:
Boyle’s Law explains why a scuba diver’s air supply decreases faster at depth. As the pressure increases, the air in the tank compresses, and the volume of air available to breathe shrinks. This is why divers need to carefully calculate their air consumption, especially on deeper and longer dives.
Decompression Sickness:
Henry’s Law is crucial to understanding decompression sickness (DCS), also known as “the bends.” As a diver ascends, the pressure surrounding them decreases, leading to the release of dissolved nitrogen from their blood and tissues. If the ascent is too rapid, nitrogen bubbles can form in the bloodstream, causing a range of symptoms, from joint pain to paralysis.
Nitrogen Narcosis:
Henry’s Law also explains the phenomenon of nitrogen narcosis. At depth, the increased partial pressure of nitrogen in the air a diver breathes causes nitrogen to dissolve into the blood and tissues, affecting the nervous system and leading to symptoms similar to intoxication, such as decreased judgment, impaired coordination, and euphoria.
Equipment Considerations:
Gas laws are also relevant to various aspects of diving equipment:
- Buoyancy Compensators (BCDs): These devices rely on the principle of Boyle’s Law to adjust buoyancy as a diver descends and ascends.
- Dive Computers: These devices use algorithms that incorporate gas laws to calculate safe dive profiles, considering depth, time, and air consumption.
Gas Laws: Your Safety Guide
Understanding and respecting these gas laws is essential for safe and enjoyable scuba diving. Dive training courses emphasize the importance of these principles and equip divers with the knowledge and skills they need to make informed decisions:
-
Plan Your Dives: Dive within your limits, consider your certification level, the depth and duration of your dives, and the prevailing conditions. It’s critical to choose dives that suit your experience and skill level.
-
Respect the Laws: Avoid rapid ascents, which could lead to decompression sickness. Pay attention to your air consumption, and make sure you have enough air to safely complete your dive.
-
Monitor your Dive Computer: Dive computers provide real-time information about depth, time, air consumption, and decompression status. They function as crucial safety tools, helping divers stay within safe limits.
-
Stay Informed: Consult reputable sources and engage with experienced divers to stay up-to-date on the latest recommendations and safety information.
Gas Laws And Scuba Diving Answer Key Pdf
Your Dive into Deeper Understanding
Learning about gas laws opens a new window into the underwater world. It gives you an appreciation for the dynamics at play beneath the surface, and a deeper understanding of the challenges and rewards of scuba diving. So, the next time you descend below the waves, remember the silent laws governing the air you breathe and the water around you.
Embrace the science behind scuba diving, and your underwater adventures will become even more rewarding and unforgettable.






