Imagine yourself in a bustling chemistry lab, surrounded by beakers filled with vibrant liquids. You’re not simply observing – you’re actively participating in the intricate dance of molecules, guided by the precision of a burette and the watchful eye of an indicator. This, my friend, is the world of acid-base titration, a fascinating experiment that unveils the secret language of chemistry.
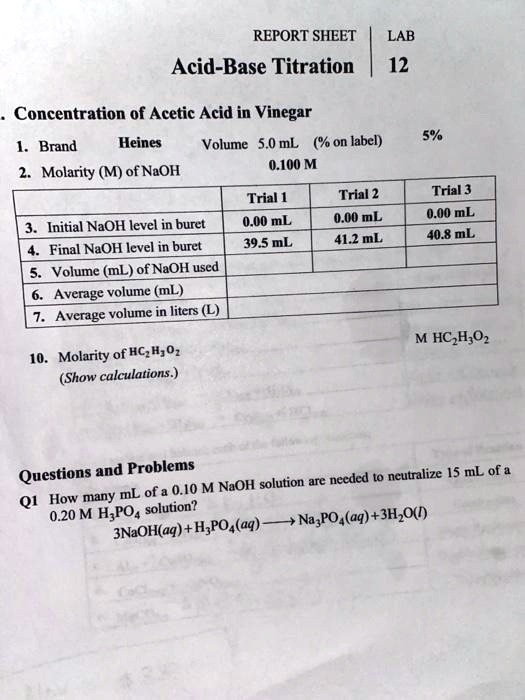
Image: aylinmeowstuart.blogspot.com
Acid-base titration is a cornerstone of analytical chemistry, a powerful technique used to determine the unknown concentration of a solution. It involves carefully adding a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until a specific endpoint is reached. This endpoint, often marked by a dramatic color change, signals that the reaction between the acid and base is complete, allowing us to calculate the concentration of the unknown solution.
Exploring the Unseen World of Titration
Titration is more than just a laboratory procedure; it’s a gateway to understanding the fundamental principles that govern chemical reactions. At its core, it explores the intricate relationship between acids and bases, those chemical opposites that attract and react in a thrilling dance of neutralization.
To truly grasp the magic of titration, we delve into its fascinating history. Titration techniques, in their rudimentary form, can be traced back to the 18th century, with pioneers like French chemist Antoine-Laurent Lavoisier and Swedish chemist Carl Wilhelm Scheele laying the groundwork for this crucial analytical method.
The development of titration was intimately tied to the burgeoning understanding of acids and bases, a field that saw the pioneering work of Svante Arrhenius, Johannes Brønsted, and Thomas Lowry. Their theories, which defined acids and bases in terms of their ability to donate or accept protons, laid the foundation for our modern understanding of acid-base reactions.
The Science Behind the Spectacle: Understanding Acid-Base Reactions
At the heart of acid-base titration lies the captivating concept of neutralization. Acids, those chemical culprits responsible for sour tastes, are characterized by their ability to donate protons (H+ ions), while bases, with their characteristic bitter flavors, readily accept these protons.
When an acid and a base react, they neutralize each other, forming salt and water. This chemical ballet involves a delicate balance of protons, with the acid surrendering its protons to the base, leading to a stable, neutral solution.
This neutralization reaction is the driving force behind acid-base titration. By carefully adding a known concentration of acid or base to a solution of unknown concentration, we can utilize the stoichiometry of the reaction to calculate the unknown concentration.
A Visual Journey: Titration in Action
Imagine a clear flask containing a solution of unknown concentration – our mystery analyte. We meticulously add a solution of known concentration, our trusty titrant, from a burette, a slender glass tube with a graduated scale.
As the titrant drips into the analyte, a dramatic change occurs – the solution’s color changes abruptly. This color change signals that we’ve reached the endpoint, the point at which the acid and base have completely neutralized each other.
This endpoint is not a mere coincidence; it’s carefully orchestrated by the use of an indicator, a substance that changes color in response to changes in pH. Indicators are clever molecules that are sensitive to the presence of excess acid or base, acting as a visual cue to signal when the reaction is complete.

Image: www.coursehero.com
Unveiling the Secrets: Calculations and Data Analysis
The data collected during titration, like the volume of titrant used and the concentration of the analyte, can be used to calculate the unknown concentration of the solution. The key lies in understanding the stoichiometry of the reaction, the precise mole ratios of the reactants and products.
Titration calculations hinge on the concept of equivalence point, the theoretical point at which the moles of acid and base are exactly equal. This theoretical point is often very close to the endpoint observed in the actual experiment.
By understanding the relationship between the equivalence point, the endpoint, and the stoichiometry of the reaction, we can determine the concentration of the unknown analyte with remarkable precision.
Titration’s Impact: Beyond the Lab
Titration, far from being an esoteric laboratory technique, permeates various aspects of our lives. It plays a vital role in various industries, including:
- Food and Beverage: Ensuring the quality of food products, measuring the acidity of fruit juices, controlling the pH of dairy products.
- Medicine and Pharmaceuticals: Developing and manufacturing medicines, maintaining the correct pH levels in drug formulations, analyzing the composition of drugs and supplements.
- Environmental Science: Monitoring water quality, analyzing soil samples, determining the levels of pollutants, and assessing the effectiveness of water treatment processes.
Unlocking the Potentials: Applications in Our Everyday Lives
Titration’s impact extends beyond scientific labs and industrial settings; it directly affects our daily lives. Whether you are enjoying a cup of coffee, indulging in a delicious meal, or taking medication, titration plays a crucial role behind the scenes.
Imagine brewing a cup of coffee. The acidity of the coffee beans is fundamental to its taste and aroma. Titration techniques are used to ensure the correct acidity level, guaranteeing a palatable and satisfying brew.
Expert Insights: Mastering the Art of Titration
Mastering titration requires an understanding of both theoretical principles and practical techniques. Dr. Emily Carter, a renowned chemist and expert in analytical techniques, offers valuable insights for aspiring titration masters:
“Titration is a powerful tool that requires meticulous attention to detail. Be precise in your measurements, ensure proper calibration of your equipment, and pay close attention to the endpoint. Don’t rush the process, and practice patience. With practice, you’ll develop a keen eye for the subtleties of titration, and your analytical skills will soar.”
Acid Base Titration Lab Write Up
Embrace the Precision: A Call to Action
The world of chemistry is a fascinating and rewarding journey. Titration, with its intricate details and precise techniques, offers a powerful lens through which to observe the intricate dance of molecules.
If you’re intrigued by the world of chemistry, embrace the challenge of titration. Seek out hands-on experiences, practice with determination, and revel in the feeling of unraveling the secrets of the chemical world. You may find yourself captivated by the artistry of this powerful analytical tool.
Go forth, experiment, and let the wonders of chemistry unfold before your eyes!





