Imagine being in a chemistry lab, surrounded by bubbling solutions and colorful reactions. You’re tasked with a worksheet, a daunting list of equations, and a request to balance them. The task seems overwhelming, a tangle of symbols and numbers. But what if you had a roadmap, a guide to navigate the world of chemical reactions, to understand their principles and conquer the art of balancing equations? This article is your compass, your key to unlocking the secrets of chemical transformation.
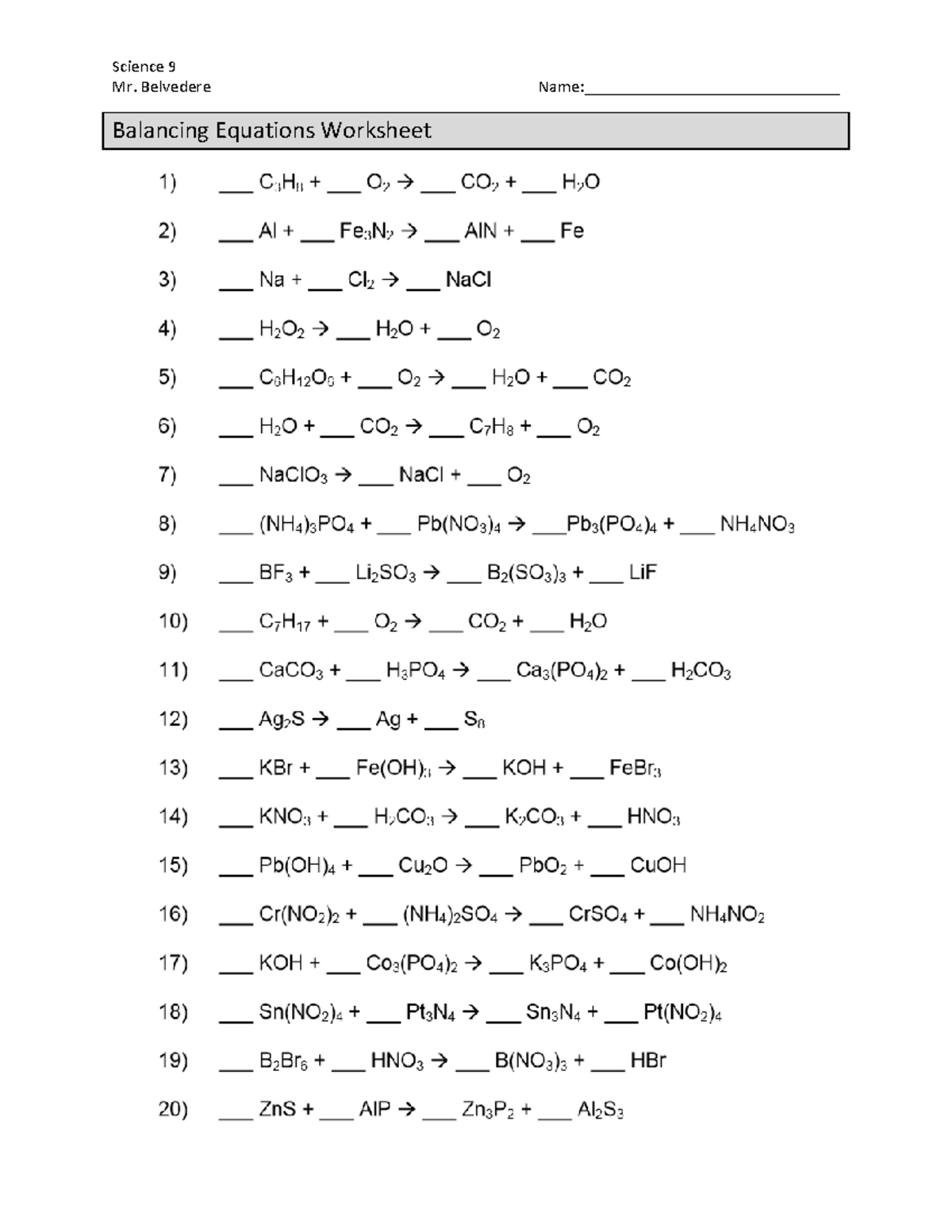
Image: classhofmann.z19.web.core.windows.net
Understanding the fundamental concepts of chemical reactions is crucial for anyone venturing into the world of chemistry. Whether you’re a student tackling a textbook or a curious mind exploring the wonders of science, grasping the basics is essential. From the simple act of rusting to the complex reactions occurring within our bodies, chemical reactions shape our world. In the following sections, we’ll delve into the fascinating world of chemical change, uncovering the different types of reactions, learning how to balance equations, and empowering you with the knowledge to master any chemistry worksheet.
The Intricate Dance of Molecules: Exploring the Types of Chemical Reactions
At their core, chemical reactions involve the rearrangement of atoms and molecules, resulting in the formation of new substances with distinct properties. Imagine a grand ballroom, where different molecules waltz and interact. Some dance alone, while others pair up, rearranging and transforming as they move. Understanding these dance moves, these distinct types of reactions, is crucial to mastering the art of chemistry.
1. Synthesis Reactions: Building New Structures
Imagine a bustling construction site, where bricks and mortar are combined to create a sturdy building. Synthesis reactions are similar, combining simpler molecules to form more complex ones. The classic example is the formation of water: two molecules of hydrogen (H2) dance with one molecule of oxygen (O2) to create two molecules of water (H2O).
Equation: 2H2 + O2 → 2H2O
2. Decomposition Reactions: Breaking Down Molecules
Now picture a sculptor meticulously dismantling a large block of marble to create a beautiful statue. Decomposition reactions are akin to this process, breaking down complex molecules into simpler ones. Think of the electrolysis of water: applying an electric current splits water into hydrogen and oxygen gas.
Equation: 2H2O → 2H2 + O2

Image: lessonlistcraig99.z21.web.core.windows.net
3. Single Displacement Reactions: A Molecular Swap
Imagine a lively dance floor where two dancers switch partners mid-dance. Single displacement reactions involve a similar exchange, where a more reactive element replaces another in a compound. Take the reaction of zinc with hydrochloric acid: zinc displaces hydrogen from the acid, forming zinc chloride and releasing hydrogen gas.
Equation: Zn + 2HCl → ZnCl2 + H2
4. Double Displacement Reactions: A Partner Swap
Picture a grand ball where partners exchange places, creating new dance pairs. Double displacement reactions involve the exchange of ions between two reactants, leading to the formation of new compounds. Consider the classic reaction of sodium chloride with silver nitrate: silver chloride precipitates out, leaving sodium nitrate in solution.
Equation: NaCl + AgNO3 → AgCl + NaNO3
5. Combustion Reactions: The Art of Burning
Imagine a cozy fireplace, where logs crackle and release heat and light. Combustion reactions involve the rapid reaction of a substance with an oxidant, usually oxygen, producing heat and light. The burning of fuels like wood, propane, and gasoline are all examples of combustion reactions.
Equation: CH4 + 2O2 → CO2 + 2H2O
Balancing the Equation: The Art of Chemical Harmony
Understanding chemical reactions is one thing, but mastering the art of balancing equations is essential to understanding the true nature of these transformations. Balancing an equation ensures that the number of atoms of each element on both sides of the equation is equal, upholding the law of conservation of mass. Imagine a skilled chef meticulously adding ingredients to their recipe, ensuring the perfect balance of flavors. That’s what balancing equations is all about: ensuring the right amount of each element is present in the chemical reaction.
The Steps to Balancing: A Recipe for Success
-
Write out the unbalanced equation: Begin by writing the chemical formulas of the reactants and products involved in the reaction.
-
Count the atoms: Tally the number of atoms of each element on both sides of the equation.
-
Adjust coefficients: Use coefficients, numbers placed before the chemical formulas, to adjust the number of molecules of each reactant and product. The coefficients must be whole numbers, ensuring that the same number of each element is present on both sides.
-
Verify balance: Double-check that the number of atoms of each element matches on both sides of the equation.
Example: Balancing the combustion of methane (CH4)
**Unbalanced:** CH<sub>4</sub> + O<sub>2</sub> → CO<sub>2</sub> + H<sub>2</sub>O
**Balanced:** CH<sub>4</sub> + 2O<sub>2</sub> → CO<sub>2</sub> + 2H<sub>2</sub>OBalancing equations is a process of trial and error, but with practice and understanding, it becomes second nature.
Unlocking the Secrets of the Answer Key: A Gateway to Chemical Mastery
Imagine finally reaching the end of your worksheet, the answer key, the key to understanding your mastery of chemical reactions. Each balanced equation is a testament to your understanding of the intricate dance of molecules. The answer key, with its carefully balanced equations, is not just a guide to right answers, but a window into the world of chemical transformation.
From Worksheets to Real-World Applications: The Importance of Balancing
The knowledge you gain from analyzing worksheet equations and mastering the balancing process extends far beyond the classroom. Balancing equations is foundational to understanding countless real-world applications, from industrial synthesis to understanding the chemistry of life itself.
-
Industrial Chemistry: Balancing equations guides chemists in designing processes for producing essential products like fertilizers, plastics, and pharmaceuticals.
-
Environmental Science: Balancing equations helps researchers understand the environmental impact of reactions, such as the burning of fossil fuels, and develop strategies for reducing pollution.
-
Biological Chemistry: Balancing equations allows biologists to decipher the intricate web of chemical reactions occurring within living organisms, from photosynthesis to the breakdown of food for energy.
Harnessing the Power of Understanding: Tips for Success
To truly conquer the world of chemical reactions, it’s not enough to just memorize equations. You need to develop a deep understanding of the principles behind them, a sense of intuition for the dance of molecules. Here are some key tips:
-
Practice, Practice, Practice: Just like learning a new musical instrument, proficiency in balancing chemical equations comes from consistent practice. Work through numerous examples, challenging yourself with different types of reactions and increasing complexity.
-
Visualize the Process: Imagine the atoms rearranging, the molecules colliding and forming new bonds. This visual approach can help solidify understanding and make balancing equations less abstract.
-
Embrace Mistakes as Learning Opportunities: Don’t be discouraged by incorrect answers. Mistakes are a sign that you’re pushing yourself to learn, to grow your understanding of chemical reactions. Analyze your errors, identify your weak points, and revisit the concepts to solidify your grasp.
Types Of Reactions Worksheet Then Balancing Answer Key
The Journey Continues: A Call to Action
The world of chemical reactions is vast and intricate, full of exciting discoveries and applications. This article is merely a starting point, a stepping stone on your journey to mastering the subject. Continue to explore, experiment, and challenge yourself with new and challenging problems. Embrace the wonder of chemical transformation. The key to unlocking its secrets lies in your own curiosity, perseverance, and dedication to understanding the dance of molecules.





