Imagine a solid cube of ice, innocently resting in a glass. Then, as the sun shines down on it, it begins to melt, transforming into liquid water. This simple change, from solid to liquid, is what we call a phase change. But the journey doesn’t end there. Heat the water further, and it will transition into a gas, vaporizing into the air. This fascinating journey of matter, shifting between solid, liquid, and gas, is an integral part of our world, driving processes ranging from weather patterns to the formation of mountains.
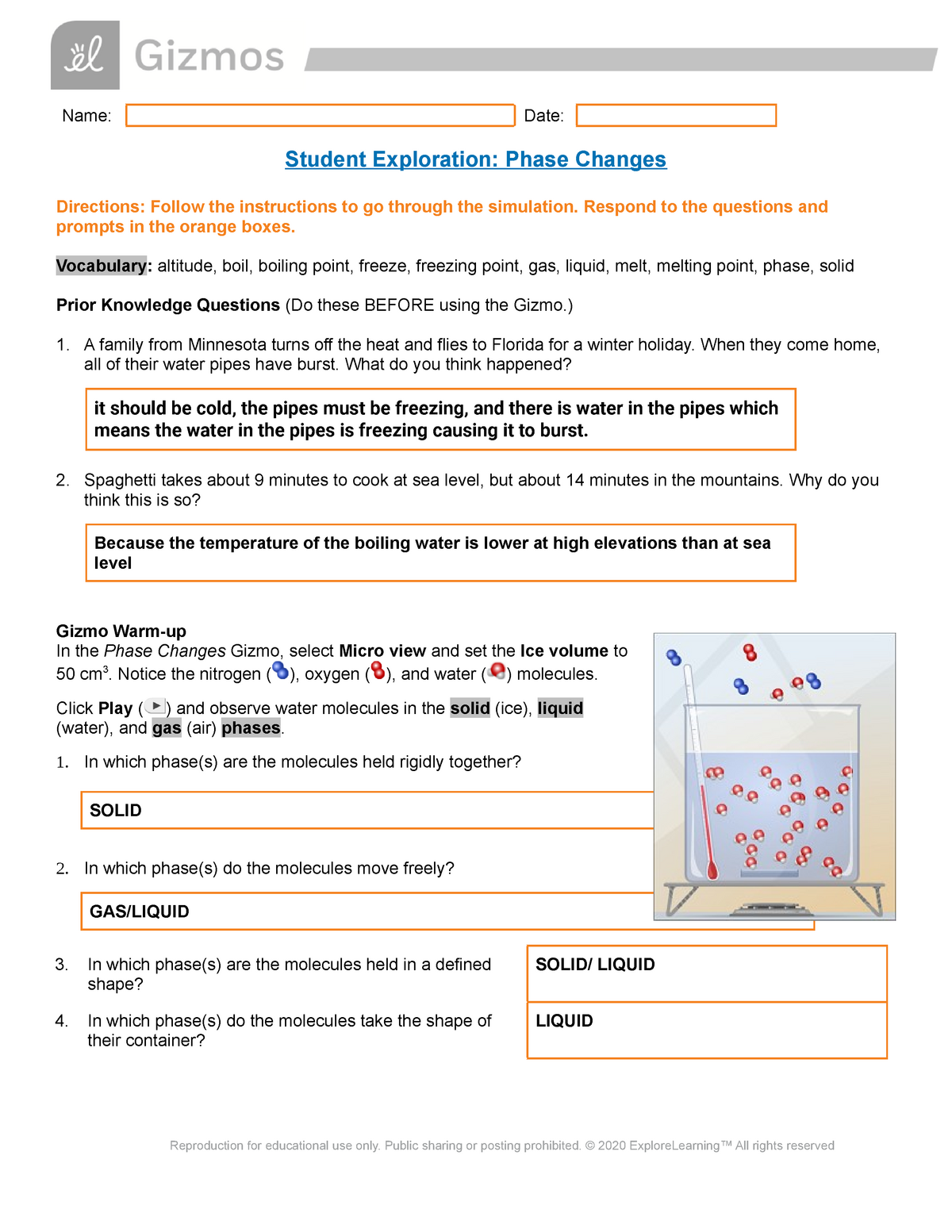
Image: jarrettgroward.blogspot.com
Understanding phase changes isn’t just about the simple observation of ice melting. It’s delving into the microscopic world of molecules, their movement, and how energy influences their arrangement. Exploring these concepts can be an exciting adventure, particularly with the help of interactive tools like the ‘Phase Changes Gizmo.’ This virtual playground allows students to experiment, explore, and gain a deeper understanding of how temperature and pressure influence the behavior of matter, making this complex topic approachable and engaging.
A Glimpse into the World of Molecules
Before we delve into the intricacies of the Gizmo, let’s first understand the foundation of phase changes: the movement and arrangement of molecules. In a solid, molecules are tightly packed together, vibrating in fixed positions, creating a rigid structure. Liquids, on the other hand, have more freedom. Molecules can move around, although they are still relatively close to each other. In gases, molecules have a lot of freedom and move rapidly, experiencing vast distances between them.
The Role of Temperature and Pressure
The key players in the drama of phase changes are temperature and pressure. Increasing temperature adds energy to molecules, causing them to vibrate faster and move further apart. Pressure, meanwhile, is the force exerted by molecules. Increased pressure causes molecules to be squeezed closer together, influencing their movement and arrangement.
Consider what happens when we heat water: initially, the water molecules gain energy and begin to jostle more. As more energy is added, the bonds holding the molecules in a fixed structure weaken, allowing them to move past each other, transforming into a liquid. Continue heating, and the molecules gain enough energy to break free entirely, transitioning into a gas.
Navigating the Phase Changes Gizmo: A Journey of Discovery
Now, let’s enter the realm of the ‘Phase Changes Gizmo.’ This interactive tool allows students to control temperature, pressure, and even the type of substance, observing the resulting changes in real-time. Through these active experiments, students gain a deeper understanding of:

Image: www.coursehero.com
1. The Effect of Temperature on Phase Changes
Within the Gizmo, students can manipulate temperature, observing how it transforms a substance’s state. Imagine starting with a solid. By increasing the temperature, students can witness the solid melt into a liquid, and subsequently, the liquid boil into a gas. This visual representation of the transition between states helps students grasp the concept of energy transfer and its impact on molecular movement.
2. The Role of Pressure in Phase Changes
Pressure is another powerful factor in phase changes, and the Gizmo allows for its manipulation. By increasing the pressure, students can observe how the boiling point of a substance shifts. For instance, a substance that boils at 100°C at normal atmospheric pressure might boil at a higher temperature under increased pressure. This interactive experience helps students understand the intricate relationship between pressure, temperature, and phase changes.
3. The Variety of Substances and Their Phase Changes
The Gizmo offers a variety of substances to explore, allowing students to observe the unique behavior of different materials. They can compare and contrast the boiling points and melting points of varying substances, understanding that different compounds have different bond strengths and energy requirements for phase changes.
From Virtual Experiments to Real-World Applications
The learning gained from exploring phase changes through the Gizmo transcends the digital realm, having real-world applications in our daily lives. Here are a few examples:
- Cooking: Understanding boiling points helps us cook effectively. Knowing how pressure affects boiling points explains why food takes longer to cook at higher altitudes and why pressure cookers work so efficiently.
- Weather: Phase changes are at the heart of weather patterns. Evaporation, condensation, and precipitation are all driven by the transition of water between its phases.
- Industrial Processes: Many industrial processes, such as refining oil and generating power, rely on the principles of phase changes.
The Future of Phase Changes: Beyond the Gizmo
The world of phase changes is constantly evolving. Researchers are exploring new materials and states of matter, pushing the boundaries of our understanding. Nanotechnology, for instance, utilizes phase changes to create novel materials with unique properties.
Student Exploration Phase Changes Gizmo Answers
Conclusion: Unlocking the Wonders of Matter Transformation
The ‘Phase Changes Gizmo’ serves as a powerful tool for students, transforming the abstract concepts of phase changes into engaging, interactive experiences. Through experimentation and observation, students gain a deeper understanding of the microscopic world, laying the foundation for a deeper appreciation of our physical world. Whether it’s understanding why water boils at a lower temperature on Mount Everest or marveling at the intricate process of ice formation, the study of phase changes opens a world of wonder and discovery. So, explore, experiment, and let the magic of matter transformation take you on an exciting journey of scientific learning!





