Have you ever wondered how scientists precisely measure the strength of an acid or a base? It seems like a simple question, but the answer lies in a fascinating and elegant chemical technique called titration. This seemingly straightforward process holds the key to unraveling the secrets of acid-base reactions, and it plays a critical role in various industries, from pharmaceutical manufacturing to environmental monitoring.
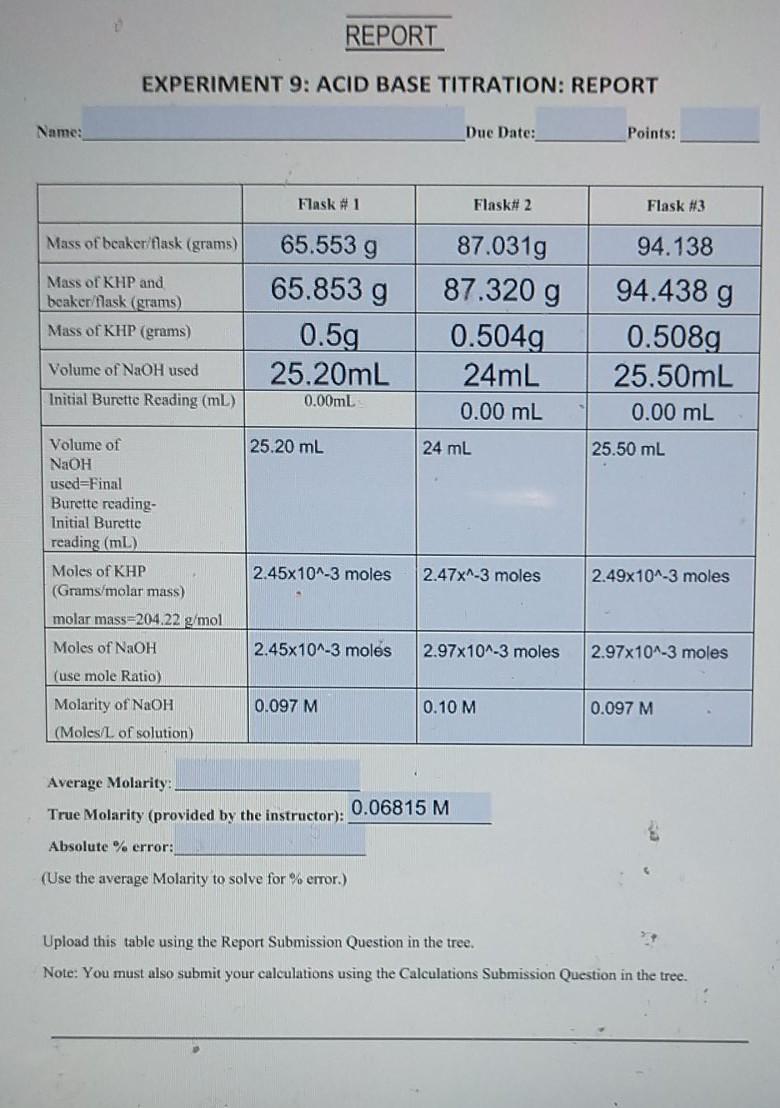
Image: www.chegg.com
Today, we’ll dive into the captivating world of acid-base titration, exploring its intricate workings, its profound applications, and its impact on our daily lives.
A Journey into the Heart of Titration: Unveiling the Chemistry Behind the Process
Titration is a fundamental analytical technique in chemistry that involves the gradual addition of a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction between them is complete. This process allows us to determine the unknown concentration of the analyte with remarkable precision.
At the core of titration lies the concept of neutralization, a chemical reaction where an acid and a base react to form salt and water. The key to successful titration is identifying the equivalence point—the point at which the acid and base have completely reacted, leaving a neutral solution. Titration relies on the careful measurement of volumes, and the equivalence point is often detected using an indicator, a substance that changes color when the pH of the solution reaches a specific point.
Demystifying the Titration Process: A Step-by-Step Guide
Imagine a scientist meticulously adding a drop of a colored solution to a flask, watching for a subtle color change. This is a simplified illustration of a titration experiment. Let’s break down the process into manageable steps:
-
Preparation: We start by preparing a solution of known concentration, the titrant, and a solution of unknown concentration, the analyte. The analyte might be a weak acid like vinegar or a strong base like sodium hydroxide.
-
Titration: The titrant is carefully added to the analyte using a burette, a graduated glass tube that allows us to control the volume of the added solution precisely. As the titrant is added, we monitor the pH change using an indicator or a pH meter.
-
Equivalence Point: The equivalence point is reached when the acid and base have completely neutralized each other. This endpoint is typically detected by a sudden color change in the solution if an indicator is used or by a sharp change in the pH reading if a pH meter is used.
-
Calculations: By measuring the volume of titrant used to reach the equivalence point and knowing the concentration of the titrant, we can then calculate the concentration of the analyte using a simple formula.
Unlocking the Applications of Titration: A Gateway to Diverse Industries
Titration’s power lies not only in its simplicity but also in its wide-ranging applications. Here are a few examples of how this powerful technique shapes various industries:
-
Pharmaceutical Industry: Titration plays a crucial role in quality control, ensuring the accuracy of drug formulations. By precisely measuring the concentration of active ingredients, pharmacists can guarantee the potency and safety of medications.
-
Environmental Monitoring: Acid-base titration is essential for monitoring water quality by determining the acidity or alkalinity of water samples. This helps ensure the safety of drinking water and protect aquatic ecosystems.
-
Food and Beverage Industry: Titration helps manufacturers ensure the quality and safety of food and beverage products. For instance, it’s used to determine the acidity of vinegar, the pH of dairy products, and the levels of certain preservatives in food items.
-
Chemical Research and Development: Titration is an indispensable tool for research chemists. It allows them to study the kinetics and mechanisms of acid-base reactions, leading to the development of new materials and products.

Image: yazminewadiaz.blogspot.com
Mastering the Art of Titration: Practical Tips and Insights
Titration, though seemingly simple, requires patience and attention to detail. Here are a few practical tips to enhance your titration skills:
-
Ensure Proper Calibration: Always calibrate your equipment, such as burettes and pH meters, before conducting any titration experiment. This ensures accuracy in your measurements.
-
Choose the Right Indicator: Selecting the right indicator for your specific titration is essential. The indicator should change color at the equivalence point of your titration reaction.
-
Maintain Constant Temperature: Temperature fluctuations can affect the reaction rate and the accuracy of your measurements. Ensure that your titration is conducted at a constant temperature.
-
Practice Makes Perfect: Like any skill, mastering titration takes practice. Perform several titrations to familiarize yourself with the process and gain confidence in your ability to obtain accurate results.
Lab Report On Acid Base Titration
https://youtube.com/watch?v=cOH7icsQz44
Titration: A Gateway to Understanding the Chemical World
Titration is a powerful tool that unlocks a deeper understanding of chemical reactions and the composition of substances. This simple yet elegant technique serves as a doorway to a fascinating world of chemistry, offering insights into the world around us.
Whether you’re a seasoned chemist or just embarking on a journey of scientific exploration, understanding titration will empower you with a deeper appreciation for the intricate mechanisms that govern our chemical world. So, embrace the art of titration, and let its precision guide you to new discoveries.






